INTRODUCTION
Intrinsic aging, a natural process, leads to structural and functional decline in the skin; it is characterized by re-ductions in collagen, elastin, chondroitin, and hyaluronic acid.1–3 These changes and other age-related factors contribute to impaired barrier function and decreased skin hydra-tion.2,4–6 Clinical manifestations of skin aging, including dryness, uneven pigmentation, reduced elasticity, and wrinkling, have prompted patients to seek treatments to enhance their skin’s appearance.7,8 Over the past decade, a diverse range of nonsurgical procedures has emerged as viable alternatives to surgical interventions. However, these less-invasive procedures have been associated with suboptimal efficacy, inconsistent clinical outcomes, and a shorter duration of tightening effects.9 Several studies have demonstrated that the acoustic energy from high-intensity focused ultrasound (HIFU) penetrates tissues more deeply than laser or radiofrequency radiation.9
HIFU offers a promising nonablative approach for skin resurfacing.10 By selectively targeting deeper dermal layers, it can induce tissue remodeling without damaging the epidermis, thus minimizing downtime.11 This method uses high-frequency ultrasound energy to create precisely localized thermal lesions, leading to collagen contraction and volume reduction.11 Furthermore, HIFU causes heating that denatures collagen fibers and disrupts hydrogen bonds in the triple helix structure, leading to collagen contraction and reorganization, while also promoting the expression of proteins involved in elastin synthesis, resulting in increased elastin fiber density. Alam et al.12 demonstrated its safety and effectiveness for facial skin tightening, and Suh et al.13 documented clinical and histopathological improvements following HIFU treatment in Asian individuals, confirming its potential as a safe, effective, and noninvasive procedure for this population. However, recovery time following HIFU varied among individuals, and mild side effects, such as skin redness or swelling, could persist for several days to weeks.14–15
Intradermal microwave (IDM) is designed based on the principles of local dynamic micro-massage to reach deeper layers, including the subcutaneous tissue. It uses frequencies ≥ 10 MHz and involves the rapid alternation of ultrasound waves at two distinct frequencies such as 1 and 3 MHz or 3 and 10 MHz.16 This frequency modulation results in rapid fluctuations in tissue pressure gradients, enabling dynamic control of the effects.17 Previous studies have demonstrated the efficacy of this therapy in promoting anti-inflammatory and regenerative processes in skin and subcutaneous adipose tissue. Applications include chronic wounds, acne and rosacea, injection lipolysis, radiation-induced fibrosis, postoperative wounds, ulcerative necrobiosis lipoidica, and breast reconstruction surgery.17–24
Although previous studies have investigated the individual effects of HIFU and IDM on the skin, their combined impact on aging-related facial skin changes remains uncertain. Therefore, we evaluated the effects of applying both HIFU and IDM on facial skin elasticity, density, glow, and hydration. The purpose of this study is to evaluate the combined effects of HIFU and IDM on facial skin elasticity, density, glow, and hydration in a sample of healthy women. We hypothesize that the combined application of HIFU and IDM will result in significant improvements in facial skin elasticity, density, glow, and hydration compared to baseline measurements.
METHODS
In total, 21 Korean female participants with dry skin (mean age 51.23±5.62 years; mean height 159.28±3.74 cm; mean body weight 56.42±4.19 kg; mean BMI=22.23±1.38 kg/m2), aged 37 to 64 years, were included in our study. Participants with no acute or chronic physical illnesses, including skin conditions, were included. Participants were excluded if they were of childbearing age, pregnant, breastfeeding, or using steroid-containing skin preparations or had sensitive skin and skin abnormalities, such as moles, acne, erythema, or telangiectasia. Moreover, those who had received treatment within 6 months were excluded.25 Prior to the study, written informed consent was obtained from all participants. The institutional review board of the Korea Institute of Dermatological Sciences approved the study protocol (approval no. KIDS-BDE002-INT). A priori sample size prediction was performed using G*Power 3.1.9.7 for Windows (University of Düsseldorf, Düsseldorf, Germany). The sample size was calculated based on power analysis using a large effect size (d=0.8). The results indicated that at least 15 participants would be needed to detect the effect of high-intensity dynamic micro-massage (HIDM) on facial skin elasticity, density, and hydration using a two-tailed test at a power of 80% and significance level of 0.05.26
A Ballistometer BLS780 (Dia-Stron Ltd., Andover, UK) was used to measure skin elasticity on the left cheek, eye area, nasolabial fold, and mouth corner of all participants (Figure 1). The device quantifies skin elasticity by measuring the vibration energy, and skin elasticity is expressed in the coefficient of restitution (CoR). This value reflects how well the skin surface recovers from deformation, providing insight into the skin’s elasticity. To measure skin elasticity, the device was calibrated by the manufacturer’s guidelines, adjusting settings to match the skin type and target area. Then, the probe was placed perpendicularly to the skin surface. In this study, the CoR value was compared before and after the intervention. An increase in CoR suggests improved skin elasticity.
A glossmeter (GM-268 Plus; Konica Minolta Corp., Japan) was used to measure skin radiance (glow) on the foreheads of all participants (Figure 2). This device quantifies the reflectance of light off a surface, and skin radiance is expressed in gloss units (GU). To measure skin radiance, the device was calibrated and the probe was placed perpendicular to the skin. Then, the 60° angle among the available options (20°, 60°, and 85°) was selected, and the measure button was pressed. In this study, the GU value was compared before and after the intervention. An increase in this value indicates improved radiance.
An Epsilon E100 (Biox Systems Ltd., London, UK) was used to measure skin moisture on the left cheeks of all participants (Figure 3). The device is utilized to assess hydration levels in the skin’s superficial layer, known as the stratum corneum, by measuring calibrated dielectric permittivity (dielectric constant, ε). It specifically measures the dielectric constant of the upper 5 μm of the skin, allowing for the creation of a two-dimensional hydration map of the skin's surface. To measure the hydration of skin’s superficial layer, the device was calibrated and the probe was placed perpendicular to the skin. Then, the measurement button was pressed, and data was collected. In this study, changes in moisture were compared before and after the intervention. An increase in moisture indicates improvement.
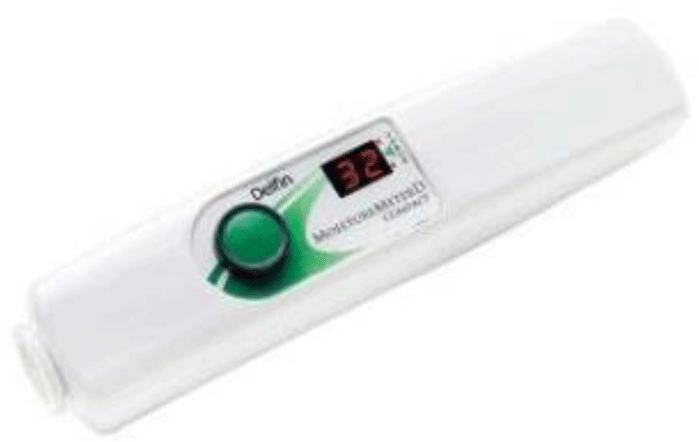
Moisture was also assessed using a Moisture Meter-D compact (Delfin Technologies, Kuopio, Finland), which does so by measuring the tissue dielectric constant (TDC) (Figure 4). The device emits a high-frequency electromag-netic wave at 300 MHz, which travels through the coaxial probe and into the skin. This wave conveys information about the dermal hydration content in the assessed tissue. To measure the hydration of skin’s deep layer, the probe of device was placed perpendicular to the skin. Then, the measurement button was pressed, and the displayed data was collected. In this study, changes in TDC were compared before and after the intervention. An increase indicates improved skin moisture.
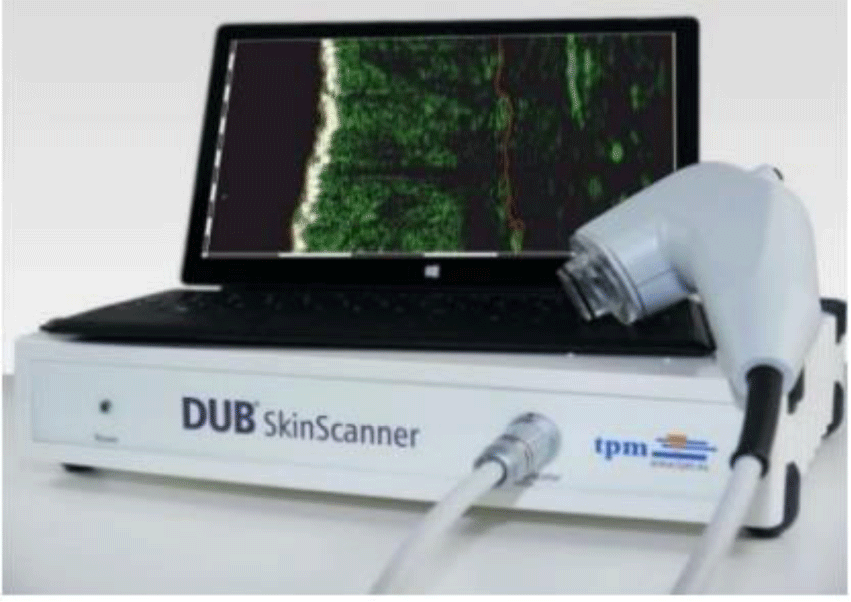
The DUB Skin Scanner (Taberna Pro Medicum, Lüneburg, Germany) was used to measure skin density (SD, %) on a 3 cm area next to the right eye corner of all participants (Figure 5). The device is utilized to obtain cross-sectional images of the skin (B-mode), and the skin density is expressed in skin density (SD, %). To measure the skin density, the probe of the device was placed perpendicular to the skin and pressed with consistent pressure. The SD data displayed on the screen was collected. In this study, SD values were recorded before and after the intervention. An increase indicates improved density.
All participants first washed their faces with the same facial cleanser and stabilized in the same environment (temperature: 22±2°C; humidity: 50±5%) for 30 minutes. Before applying HIDM, participants had their skin elasticity, density, glow, and hydration (deep and surface) measured. Then, a consistent amount of Home Esthetics Booster Gel (JLU, Ltd., Busan, South Korea) was evenly applied to the facial area. The gel was gently massaged for 10 minutes using the HIDM device (JLU, Ltd.) in IDM mode (Figure 6). Subsequently, the same procedure was repeated in HIFU mode (Figure 7). All participants received a single 20 minutes of HIDM treatment and had a 1-minute rest period between each condition. After HIDM application, skin elasticity, density, glow, and hydration (deep and surface) were re-measured. All previous skin measurements and treatments were taken at a single time point.
A Kolmogorov-Smirnov Z-test was conducted to assess the normality of continuous data. Paired t-tests were used to compare facial skin elasticity, density, glow, and hydration before and after the intervention. p-values <0.05 were considered statistically significant. Statistical analyses were performed using SPSS software (version 16.0; IBM Corp., Armonk, NY, USA).
RESULTS
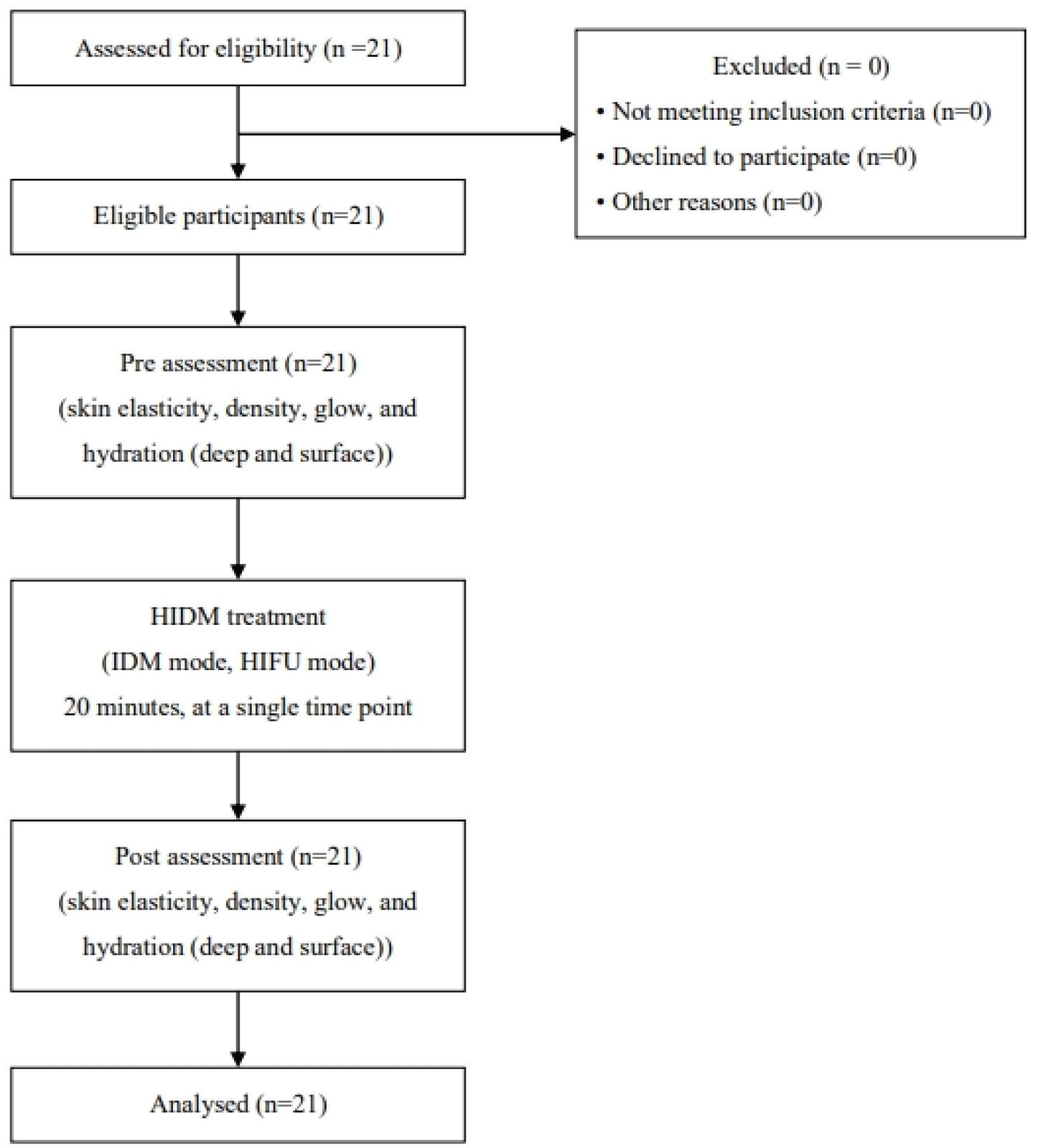
All participants completed the study with no missing data (Figure 8). Table 1 presents the results of statistical analysis. All outcome variables, including facial skin elasticity, density, glow, and hydration, were significantly higher after the interventional ultrasound compared to baseline (p<0.001).
DISCUSSION
We evaluated the effects of HIFU and IDM on facial skin elasticity, density, glow, and hydration. The findings demonstrated significant improvements in skin elasticity of cheek (1.63%), eye area (1.85%), nasolabial fold (1.69%), and mouth corner (1.63%) following the application of HIDM (HIFU mode+IDM mode). In addition, skin density (8.09%), glow (41.97%), deep hydration (283.13%), and surface hydration (26.33%) were significantly improved after applying HIDM.
The treatment significantly improved facial skin elastic-ity, particularly in the cheek, eye area, nasolabial fold, and mouth corner, presumably by stimulating collagen production and minimizing recovery time associated with collagen generation-related damage. Our results are consistent with Zhu et al.,27 who reported a significant reduction in skin tightness and volume after HIFU treatment in 20 healthy women. HIFU heats the tissue and induces thermal coagulation at a predetermined depth, stimulating the formation and remodeling of collagen.28 This inflammatory response leads to tissue damage, which triggers collagen contraction and new collagen production, resulting in skin tightening and lifting without damaging the epidermis.29 These mechanisms likely contributed to the increased facial skin elasticity observed in our study. However, it is reported that recovery time after applying HIFU varies among individuals and can last from several days to several weeks.14,15 The IDM mode of HIDM may have helped regulate HIFU-induced inflammation, promoting elasticity recovery. The vibrations generated by the IDM mode of HIDM stimulate heat shock proteins, supporting the wound-healing pro-cess.30–31
From a cellular and molecular perspective, HIFU regulates the length of cilia in adipose-derived stem cells (ASCs), promoting adipogenesis. This process involves the modulation of inflammatory cytokines and the upregulation of heat shock proteins.32 Heat shock proteins are cellular proteins produced in response to stress, including elevated temperatures. These proteins protect cells by aiding the correct folding of newly synthesized proteins and preventing the aggregation of misfolded proteins.33,34 Previous studies have demonstrated the significant anti-inflammatory and regenerative effects of heat shock protein therapy on skin and subcutaneous adipose tissue in various conditions.18–24 HIFU enhances facial skin elasticity by inducing collagen contraction and stimulating the formation of new collagen. Furthermore, IDM reduces the recovery period by regulating HIFU-related inflammation, facilitating the restoration of skin elasticity. Therefore, the two together effectively improve elasticity in a short timeframe. Although we did not compare the individual effects of HIFU and IDM, their simultaneous application enhances facial skin elasticity more effectively than either alone, and improves patient satisfaction compared to individual use. Therefore, HIDM treatment is proposed as a safe and fast way to enhance facial skin rejuvenation with reduced recovery time and side effects.
Skin density also improved, suggesting that HIFU stimulated the remodeling of collagen and the synthesis of elastin fiber in the dermis, which plays a critical role in skin density, whereas IDM may have reduced recovery time from potential damage, consistent with prior studies. In previous studies that compared the effects of UVB irradiation alone and combined UVB irradiation and HIFU in mouse models, the latter group experienced a significant increase in collagen remodeling and elastin fiber formation, resulting in enhanced skin density compared to controls.35 In molecular-level studies, HIFU has been reported to increase the expression of TGF-β (Transforming Growth Factor-Beta) signaling molecules and inhibit MMP3 (Matrix Metalloproteinase 3), which stimulates collagen remodeling and elastin synthesis.35,36 Proteins involved in elastin synthesis, which play a crucial role in skin density, are naturally produced only during wound healing, making elastin fiber replacement challenging.37–39 Currently, few treatments can effectively increase the synthesis of elastin fiber.40 Previous studies have demonstrated that HIFU promotes the expression of proteins involved in elastin synthesis, leading to increased elastin fiber density in aged skin.35 This suggests that HIFU may be an ideal method for skin rejuvenation by stimulating the synthesis of collagen and elastin fiber. Furthermore, HIFU plays a crucial role in reorganizing collagen patterns and inducing the synthesis of elastin fiber in tissue, increasing the structural density and enhancing the firmness of skin. In addition, IDM may have helped reduce the recovery time associated with potential HIFU-related damage, contributing to faster overall results. As this study focused on healthy women, the observed improvements were moderate. Further studies on women with more aged and photodamaged skin may demonstrate more significant effects.
The treatment also significantly enhanced skin glow and hydration (deep and surface). This is likely attributable to increased hydrogen bonding between collagen fibers and water molecules as new collagen was formed. Water forms hydrogen bonds with collagen or other water molecules in biological systems, such as the skin. Free water refers to water that does not bind with proteins.41 In young skin, most water is bound to collagen, whereas in aged skin, it predominantly exists in free-water form.42 This reduced protein–water interaction in aged skin can be improved by HIFU. HIFU causes localized heating, leading to the denaturation of collagen fibers and the disruption of hydrogen bonds within the triple helix structure; this results in collagen contraction and reorganization. Additionally, newly generated collagen fibers are more compact and well-aligned, enhancing their binding ability with water molecules.43 Furthermore, HIFU can enhance the production of collagen and hyaluronic acid by increasing skin tempera-ture, leading to improved moisture retention in the skin.44 This enhances moisture retention, and then the IDM component of HIDM quickens recovery. This improvement in moisture naturally improves skin glow and hydration. Considering the preceding points, HIFU improved critical anti-aging indicators, including skin elasticity, skin density, glow, and hydration. Thus, HIFU will be an effective method for ani-aging treatment.
Our study had several limitations. First, it lacked histo-logical analysis for a more objective assessment of efficacy. Second, there is no control group, which makes it difficult to clearly evaluate the effects of the HIDM treatment. Third, we could not determine the effects of various treatment frequencies and durations because we focused only on the immediate effects. Finally, the study was limited to healthy women, limiting its generalizability. Although the study demonstrates significant improvements, further research is needed to evaluate the long-term effects of HIDM therapy and its efficacy across different skin types, ages, and sex. Additionally, including a control group in future studies would help clarify the extent to which HIDM outperforms other noninvasive skin treatments.
CONCLUSIONS
We evaluated the impact of interventional ultrasound therapy using HIDM (HIFU+IDM mode) on facial skin elasticity, density, glow, and hydration. Our findings indicate that HIFU stimulates collagen, elastin, and water binding, whereas the regenerative effects of IDM contribute to rapid improvement in these skin parameters. Thus, HIDM is a safe, noninvasive, and effective approach for enhancing facial skin elasticity, density, glow, and hydration.