INTRODUCTION
Hip extension is a viral movement utilized in daily life and sports activities.1 The gluteus maximus (GM), the biggest and most powerful muscle in a normally functioning human body, is tasked with executing hip extension and external hip rotation.2–6 It has three primary roles. In particular, it acts as a local and global stabilizer and serves as a global mobilizer. As a local stabilizer, the GM contributes to the stabilization of the lower back, sacroiliac joint, lumbo-sacral region, and femoral head within the acetabulum.7–10 As a global stabilizer, it controls range of motion via eccentric and/or isometric contractions across three planes of motion.11 As a global mobilizer, it produces force and power to contribute to hip extension and external rotation.12
However, the GM is susceptible to weakness and inhibition, which can negatively affect athletic performance, and is correlated with various types of injuries and chronic pain.13 GM weakness is associated with conditions such as patellofemoral pain, anterior cruciate ligament injuries, low-back pain, hamstring strains, femoral acetabular impingement syndrome, and ankle sprains. This weakness or dys-function may either contribute to or result from these injuries.14–20 Lifestyle factors, particularly prolonged sitting, can significantly reduce GM activity.21 Prolonged sitting can lead to reduced GM activation and is often accompanied by hip flexor tightness and local core weakness, resulting in an anteriorly tilted pelvis that stretches the GM and places it in a mechanically disadvantageous position.5 In addition, reciprocal inhibition of the GM due to hip flexor muscle overactivity can contribute to lower-extremity injuries.22 Pain also significantly inhibits GM function, leading to delayed and reduced muscle activation.23
Active prone hip extension is an exercise commonly used in physical therapy for patients with hip or trunk dysfunction.24 In addition, this exercise is used as a self-perturbation task to assess the stability of the lumbopelvic region. Clinically, patients with lumbopelvic dysfunction often extend or rotate excessively during prone hip extension.25 Previous research has examined muscle activation patterns during active prone hip extension. Another study showed that evaluating the movement patterns and the balance between hip and trunk muscle activity during active prone hip extension is essential for distinguishing patients with low-back pain from healthy individuals.24,26
In relation to the GM and low-back pain, bilateral GM weakness increases lumbar lordotic curve by tilting the pelvis anteriorly, thereby causing lower-back pain.25 However, there is still insufficient research on how unilateral GM weakness leads to excessive lumbopelvic rotation and, subsequently, causes lower-back pain. Previous studies commonly showed that insufficient trunk muscle activation or GM activity leads to lumbopelvic rotation during active prone hip extension.24,26 However, there is still a lack of research on whether GM strength or GM strength asymmetry causes pelvic rotation. Therefore, the current study aimed to investigate the relation of GM weakness to pelvic rotation during active prone hip extension in healthy individuals by examining the correlation between GM strength and pelvic rotation angle and between GM strength asymmetry and the difference in pelvic rotation angles on the left and right sides. It was hypothesized that GM weakness is related to pelvic rotation angle, and that asymmetry in GM strength asymmetry is related to the difference in pelvic rotation angles on the left and right sides during active prone hip extension.
METHODS
Sample size was determined a priori using G*Power (version 3.1.9), based on a power of 0.80, an alpha level of 0.05, and a correlation of 0.5. If there was a significant correlation between strength of the GM muscle and lumbar rotation during prone hip extension, a correlation value of at least 0.5 (moderate effect) could be obtained. The sample size required was at least 29. The current study included 35 male healthy participants. The anthropometric details (mean ±standard deviation) of the participants were as follows: age, 22.3±2.4 years; height 1.73±3.29 m; and weight, 74.15± 9.38 kg. The current study included participants who did not present with any neuromuscular or musculoskeletal dysfunction with lumbar spine or hip joint that could interfere with leg movements. Participants who had a hip extension angle of <10° were excluded from this study. The experimental procedures were fully explained to the participants, and each participant provided written informed consent on a form authorized by the Public Institutional Review Board (certification number: P01-202409-01-041).
The Smart KEMA tension sensor (Korea Tech Co., Ltd., Seoul, Korea) was utilized to assess isometric strength and to set the initial belt tension at 3 kgf.27 Tension sensor was measurable up to 1960 N, with an accuracy of 4.9 N and a sampling frequency of 10 Hz.27 The force signals were measured using the maximal voluntary isometric contraction of the GM via hip extension. The Smart KEMA tension sensor had an excellent intra-rater (intraclass correlation coefficient3.1>0.95) and inter-rater (intraclass correlation coefficient2.1>0.95) test reliability.28
To measure GM strength, the belt length was adjusted to assess isometric strength in the prone position, and the strap was placed on the distal thigh. The participants were instructed to extend their hips with the knees flexed to 90° to minimize force contribution of the hamstrings and thorough active insufficiency and to hold maximal strength for 5 s.22 The examiner stabilized the participant’s pelvis by manual to prevent the lumbopelvic rotation during hip extension. The participants performed hip extension against a strap to maximal voluntary isometric contraction three times. The strength of the right and left GM muscles was measured. To minimize muscle fatigue, the participants were allowed to rest for 1 min between the trials. The maximum value from the GM strength data, which was measured three times on each side, was used in the analysis. The maximum values of the collected right and left GM strength were compared, and the larger value was labeled as strongest while the smaller value was labeled as weakest to calculate GM strength asymmetry. The following formula was used to calculate GM strength asymmetry: [(strongest–weakest/strongest) × 100].29
To measure the pelvic rotation angle, a smart phone was connected to the holder of a smart phone-based measurement tool (SBMT).30 Previous research has shown that pelvic rotation measurements using the SMBT have an excellent reliability.31 The inclinometer application (clinometer level and slope finder; Paincode Software Solutions, Stephanskirchen, Germany) was calibrated by placing the SBMT on a level surface before measuring pelvic rotation. The base of the SBMT was positioned at both the posterior superior iliac spines and the inclinometer application was used to measure the pelvic rotational angle during prone hip extension.
To perform prone hip extension, the participants were instructed to lie on a table in the prone position. Each participant was instructed to perform active unilateral hip extension from neutral to 10° extension while keeping the knee extended. For each participant, the hip extension angle was defined by the placement of a bar. The hip was held in the extended position for at least 3 s. The prone hip extension was conducted twice on each side. The maximum pelvic rotation angle measured during the prone hip extension for both the right and left legs was used to examine the correlation between GM strength and pelvic rotation angle. In addition, the difference in pelvic rotation angles between the right and left prone hip extension was calculated to analyze its correlation with GM strength asymmetry.
The Kolmogorov–Smirnov test was applied to determine whether the data sets had a normal distributed. Pearson’s correlation coefficients were used to examine the correlations between GM strength and pelvic rotation, as well as between GM strength asymmetry and difference in pelvic rotation angles. The correlation effect size was interpreted as follows: r<0.1, trivial; 0.11–0.3, low; r=0.31–0.5, moderate; r=0.51–0.7, large; r=0.71–0.9, very large; r>0.9, almost perfect.32 Statistical analyses were performed using the Statistical Package for the Social Sciences for Windows version 19.0 (SPSS, Inc., Chicago, IL, the USA).
RESULTS
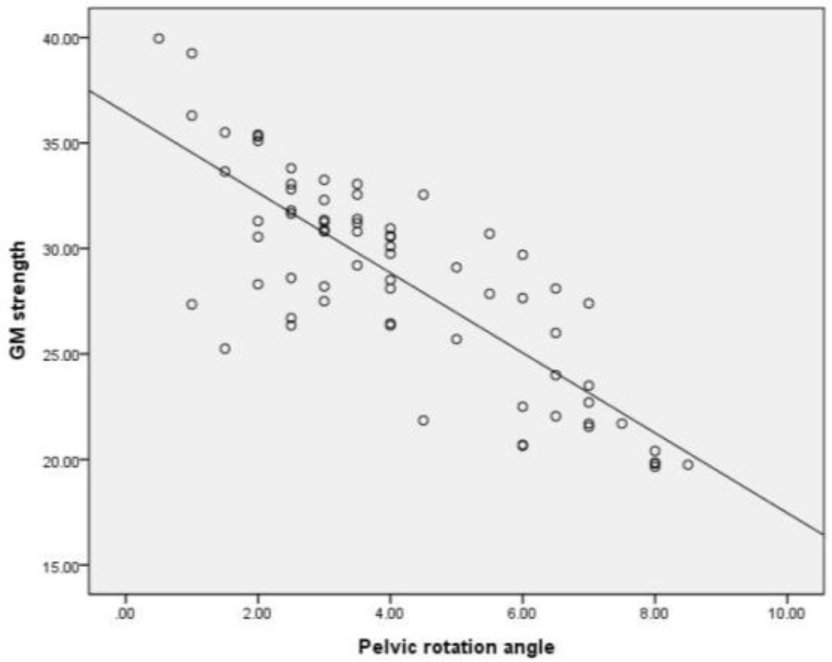
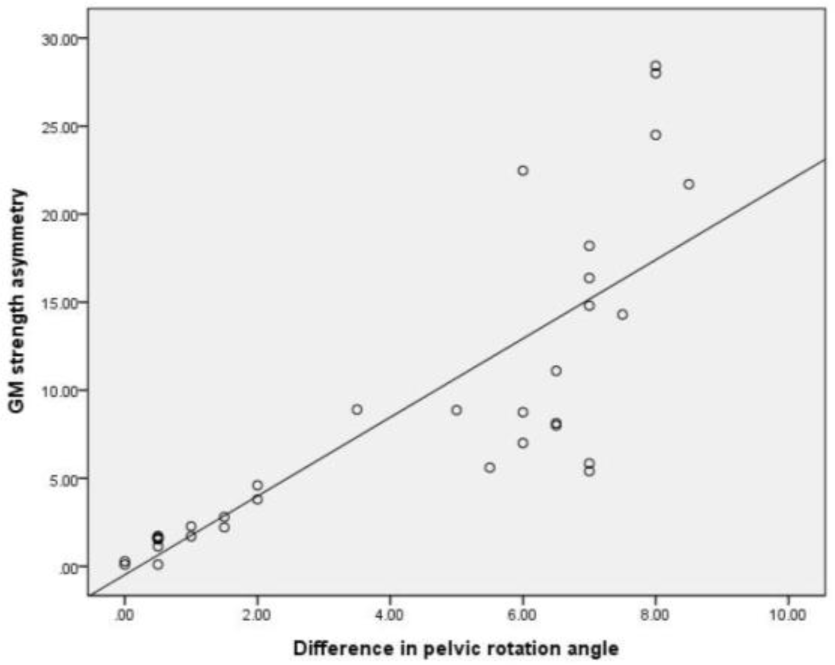
Table 1 shows the correlation coefficients between GM strength and pelvic rotation angle during prone hip ex-tension. There was a significant correlation between GM strength and pelvic rotation angle during prone hip exten-sion (r=–0.807) (Table 1, Figure 1). Table 2 shows the correlation coefficients between GM strength asymmetry and difference in pelvic rotation angles during prone hip extension. Further, a significant correlation was observed between GM strength asymmetry and difference in pelvic rotation angles during prone hip extension (r=0.825) (Table 2, Figure 2).
| Pelvic rotation angle during prone hip extension | ||
|---|---|---|
| r | p | |
| GM strength | −0.807 | 0.001 |
| Difference in pelvic rotation angle during prone hip extension | ||
|---|---|---|
| r | p | |
| GM strength asymmetry | 0.825 | 0.001 |
DISCUSSION
This study investigated the relation of GM weakness to pelvic rotation during active prone hip extension in healthy individuals. Results showed a very large negative correlation between GM strength and pelvic rotation angle during prone hip extension (r=–0.807). This finding indicates that if there is a weaker GM strength during prone hip extension, the pelvic rotation is greater.
Previous research has shown that the causes of lumbopelvic rotation during hip extension movement include faulty movement patterns of the abdominal muscle, unilateral hip flexor stiffness or shortness, and an imbalance between hip and trunk muscle activity.12,25,33 However, the results of this study indicate that GM strength also influences pelvic rotation during hip extension movement.
The GM is connected to the erector spinae and thoracolumbar fascia, contributing to the segmental stability of the lower back.12 In addition, as part of the posterior sling, it works with the hamstring, thoracolumbar fascia, opposite latissimus dorsi, and triceps to contribute to rotational trunk stabilization.33 Therefore, as shown in the results of this study, GM weakness induces compensation in the posterior sling during hip extension, leading to an increase in pelvic rotation. Consequently, GM weakness could be a contributing to not only lumbar extension and pelvic anterior tilting but also pelvic rotation.
Further, there was a very large positive correlation between GM strength asymmetry and difference in pelvic rotation angles during prone hip extension (r=0.825). This finding indicates that if there is a significant variation in muscle strength between the right and left GM, the difference in pelvic rotation angles increases more during prone hip extension in each leg. An increase in lumbopelvic rotation to one side shows a lack of ability to control this rotation, which can lead to lower-back pain or pain in the sacroiliac joint.5,33 In addition, excessive lumbopelvic rotation to one side during unilateral leg movement results in movement impairment that is often observed in lumbar rotation syndrome.25 A greater strength asymmetry between the right and left leg muscles has more impact on balance, and it increases the risk of lower-extremity injuries, potentially causing sports injuries, particularly in athletes.34,35 Therefore, overall GM strength and bilateral symmetrical strength are important for preventing pelvic rotation during prone hip extension.
The current study had few limitations. First, this study did not measure abdominal muscle activity. Hence, the effect of abdominal muscles on pelvic rotation remains unknown. Second, it only measured pelvic rotation using an SBMT, and other movements such as lumbar rotation and extension were not assessed. Third, it only included 35 healthy male participants; thus, it is challenging to generalize the results. Therefore, further studies with a larger sample size across all sexes and various age groups should be performed to investigate which factors, such as abdominal muscles, hip flexor length, and gluteus maximus strength, have a greater influence on pelvic rotation during prone hip extension.
CONCLUSIONS
There was a very large significant negative correlation between GM strength and pelvic rotation angle and a very large significant positive correlation between GM strength asymmetry and difference in pelvic rotation angles during prone hip extension. Thus, GM weakness are the contrib-uting factors of lumbopelvic rotation during hip extension movement and overall GM strength and bilateral symmet-rical strength are essential to prevent pelvic rotation.







